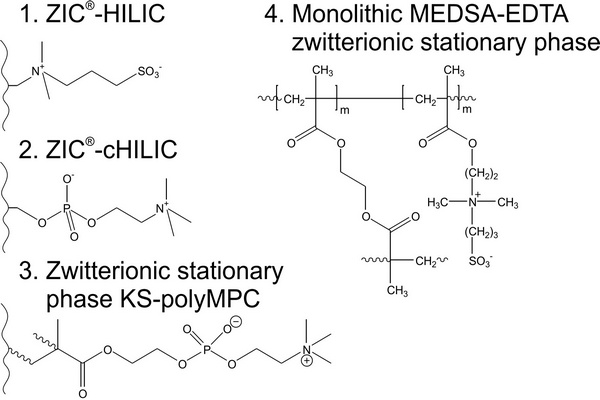
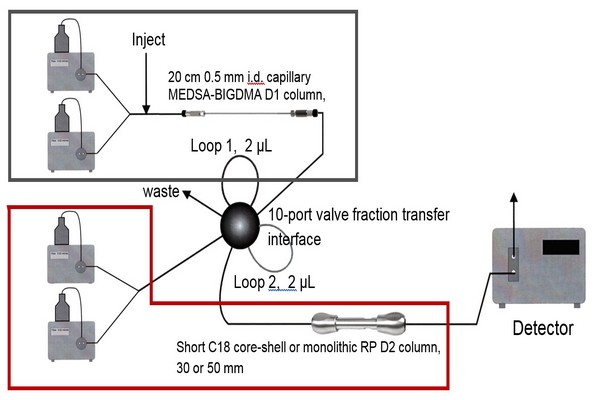
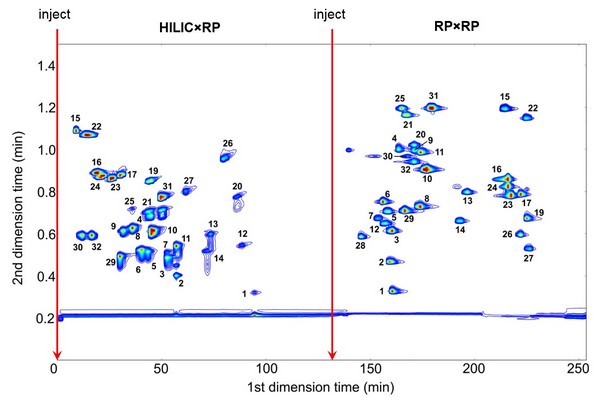
Chapter 2 - Introduction - Page 39
In reversed-phase (RP) systems, most frequently used in contemporary high-performance liquid chromatography (HPLC) practice, the stationary phase is nonpolar, usually an alkyl-silica type bonded phase, whereas the mobile phase is a mixture of one or more organic solvents and water or an aqueous buffer. As a rule, sample retention increases for more lipophilic samples and stationary phases and in more polar mobile phases; polar solutes are less strongly retained than the nonpolar ones. Very hydrophilic samples such as carbohydrates or small strongly polar compounds are weakly retained in RP-LC systems and often elute close to the column hold-up volume, so that their separation from one another and from polar matrix interferences may be difficult to accomplish, even in highly aqueous mobile phases (Pereira et al., 2009).
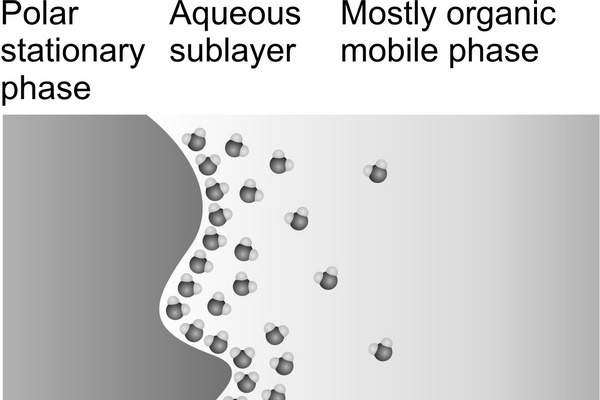
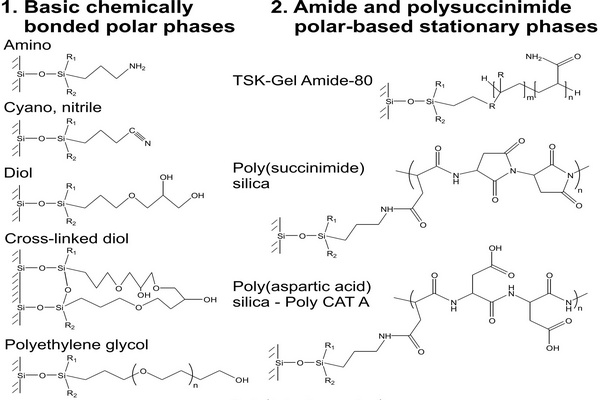
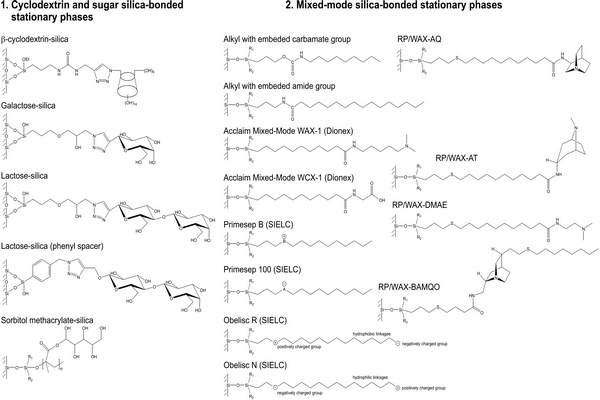
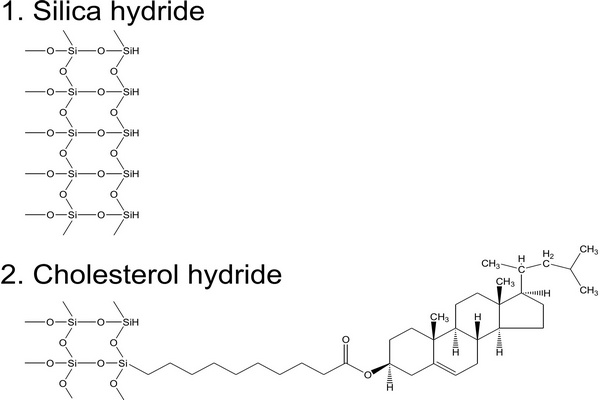
On the other hand, the stationary phase in normal-phase (NP) chromatography is more polar than the mobile phase, and opposite to RP-HPLC retention increases with increasing polarity of samples and of the stationary phase and also in less polar mobile phases. In nonaqueous mobile phases traditionally used in conventional NP (adsorption) chromatography, the retention mechanism is based on the competition between the sample and the mobile phase for localized polar adsorption centers on the adsorbent surface (Snyder et al., 2009). However, strongly polar compounds are often excessively retained in nonaqueous NP systems or are poorly soluble in nonpolar or in weakly polar organic solvents. Often, their separation on polar stationary phases can be improved by adding water to the mobile phase (Huber et al., 1984). Some water accumulates close to the polar surface, where it forms a diffuse layer more polar than the bulk aqueouseorganic mobile phase, which is less rich in water (Fig. 2.1). This approach had been occasionally used a long time before Alpert introduced the name “hydrophilic interaction liquid chromatography” (HILIC) for this separation mode (Alpert, 1983, 1990). The term “hydrophilic” refers to affinity for water. Essentially, HILIC systems can be understood as a “normal-phase stationary phase” in combination with a “reversed-phase mobile phase,” usually containing 50% or more of water.
The HILIC technique provides appropriate retention and resolution for many polar compounds, often with better separation efficiency in comparison to the RP chromatography (Gritti et al., 2010). The diffusion coefficients of ionized basic compounds in less viscous organic-rich mobile phases under HILIC conditions are approximately twice those under RP conditions, leading to improved separation efficiency (lower height equivalent of a theoretical plate, HETP) (McCalley, 2007, 2008). Another reason for the increasing popularity of HILIC is its excellent suitability for coupling to mass spectrometry (LC/MS).