
|
Wm. Craig Byrdwell, phd |
|
Resources for Lipid Analysis in the 21st Century |
|
Sphingomyelin Synthesis |
|
To contact me: |
|
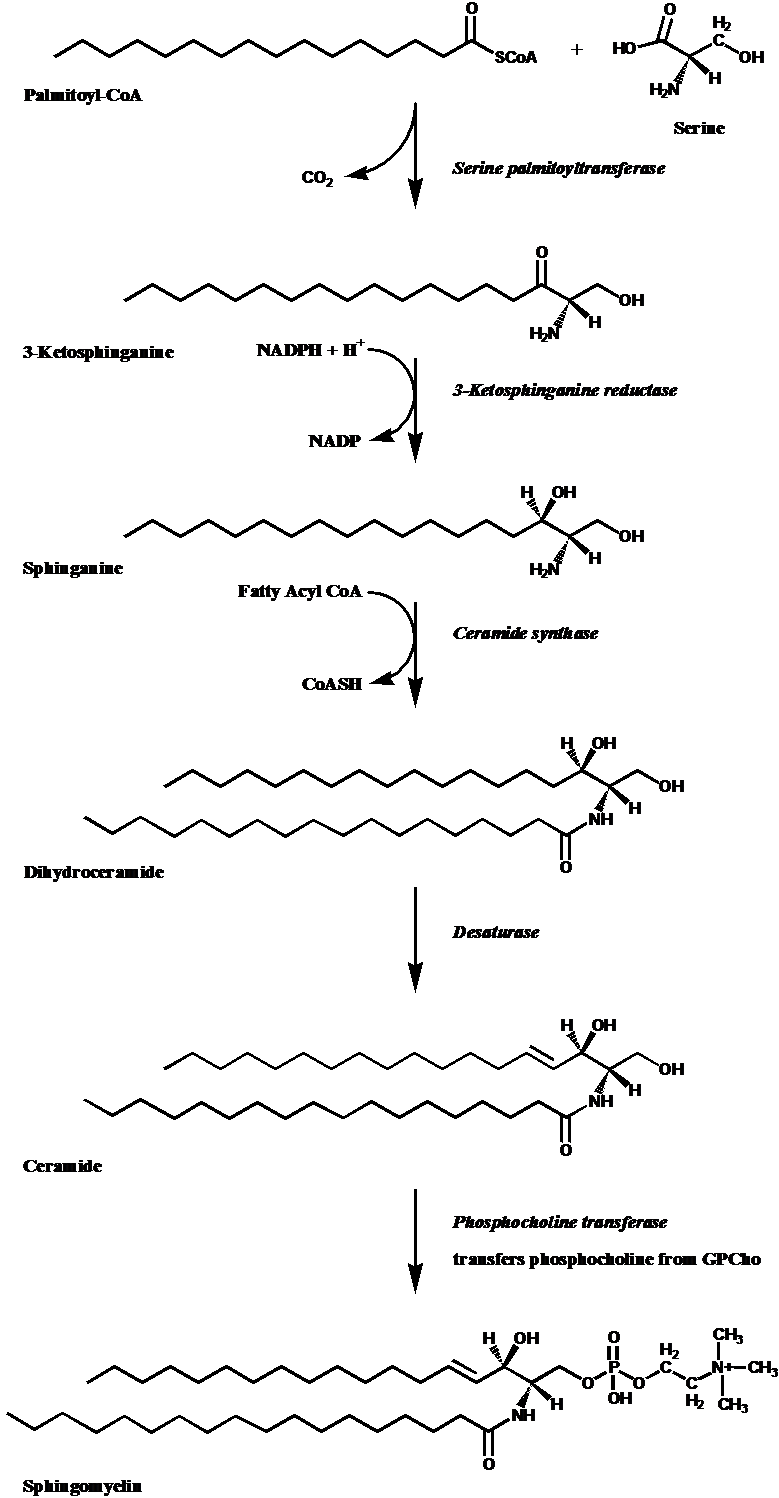
This Page shows the biological synthesis of Sphingomyelin molecules. Synthesis occurs on the cytoplasmic leaflet of the endoplasmic reticulum.1 |
|
Copyright © 2005-2011 Byrdwell.com
The figures and tables on this website are copyrighted in 2005-2011 by William Craig Byrdwell. If you use these tables please reference www.Sphingomyelin.com |
|
1. Merrill, A.H. and Sweeley, C.C., Sphingolipids: Metabolism and Cell Signalling, in Biochemistry of Lipids, Lipoproteins and Membranes, Vance, D.E. and Vance, J. (Eds.) Elsevier, Amsterdam, the Netherlands, pp. 309-339, 1996. |
|
Palmitoyl Coenzyme A is combined with a serine molecule through action of the enzyme Serine palmitoyltransferase to yield 3-Ketosphinganine. A molecule of CO2 is liberated in the process. |
|
The ketone moiety in 3-Ketosphinganine is reduced to an alcohol by the enzyme 3-Ketosphinganine reductase to yield the sphinganine molecule. Nicotine adenine dinucleotide phosphate (NADPH) provides the hydrogen atoms for the reduction. |
|
A fatty acyl chain from Fatty Acyl Coenzyme A is added to the Sphinganine nitrogen by action of the enzyme Ceramide synthase, to yield Dihydroceramide. |
|
A Desaturase enzyme acts on Dihydroceramide to produce a site of unsaturation (double bond) at the 4,5 position of the backbone. The result is a Ceramide molecule, which forms the basis of most Sphingolipids. |
|
A phosphocholine head group is transferred from PhosphatidylCholine (Glycerophosphocholine) onto Ceramide by the enzyme Phosphatidylcholine transferase. This yields the final product, Sphingomyelin. Sphingomyelin differs from Dihydrosphingomyelin by the presence of the 4,5 trans double bond. |
|
Phone: 301-504-9357 Fax: 301-504-8314
Please bring any inaccuracies in these pages to my attention at: E-mail: Byrdwell@Byrdwell.com |