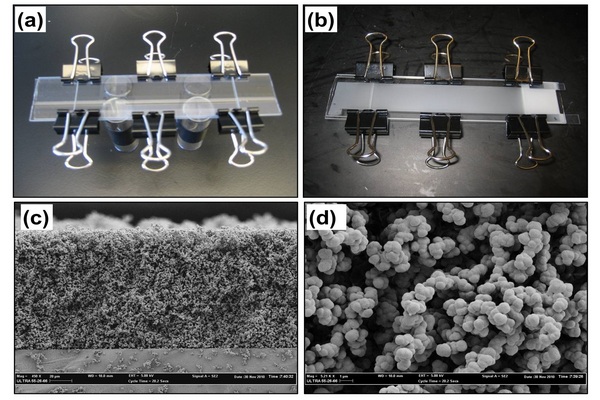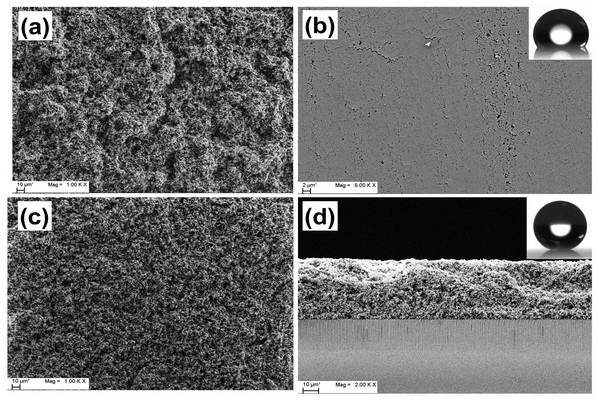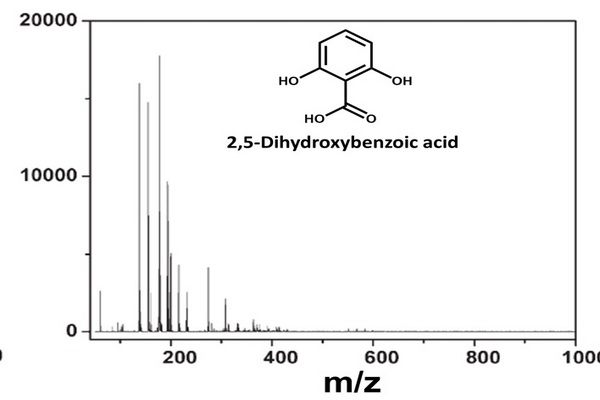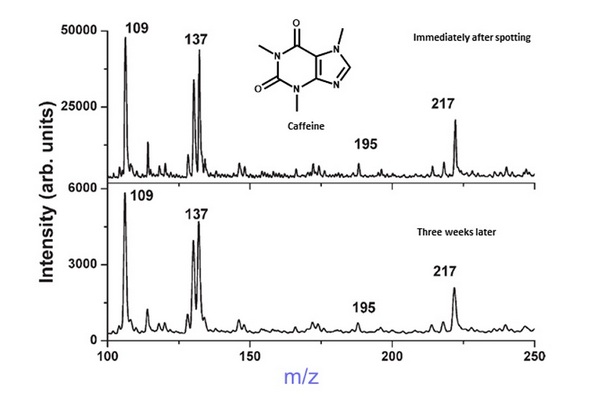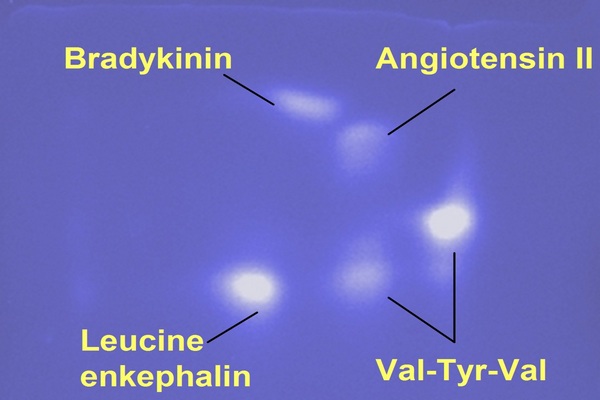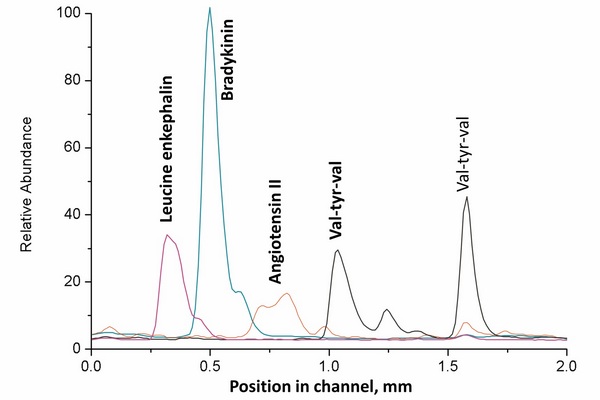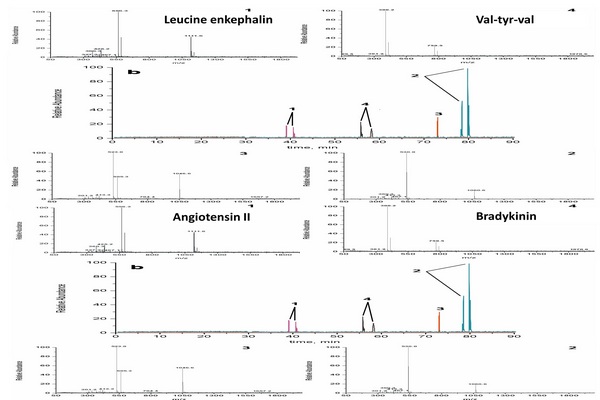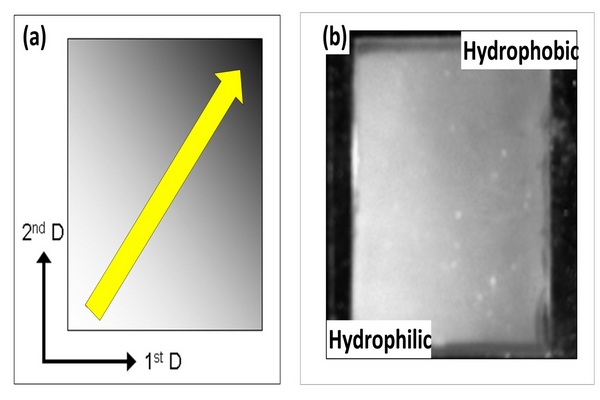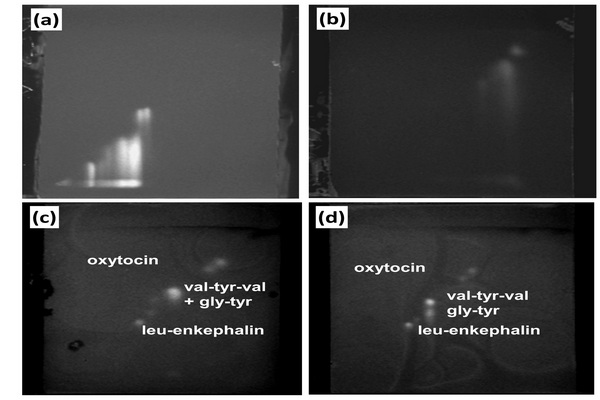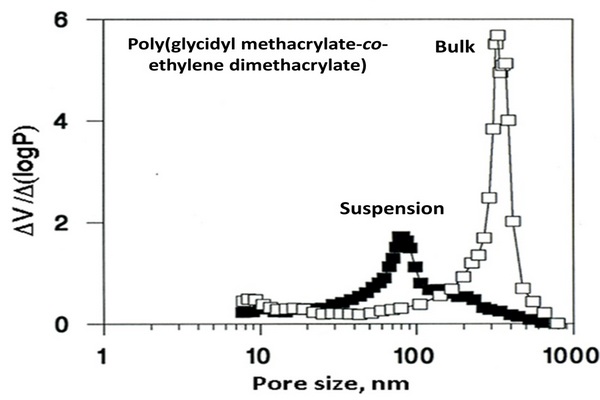
Figure 5.1
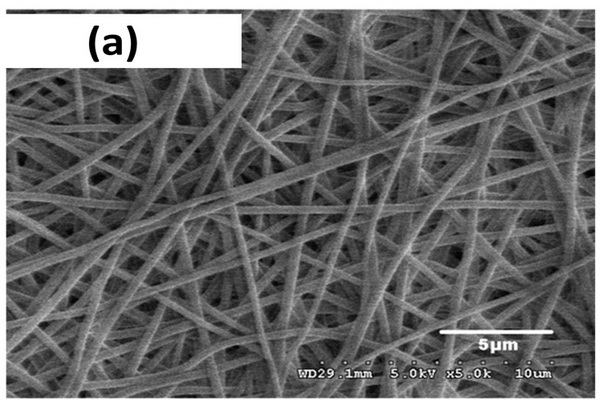
Differential pore size distribution plots of the poly(glycidyl methacrylate-co-ethylene dimethacrylate) beads and monolith prepared from the same polymerization mixture consisting of glycidyl methacrylate (24%), ethylene dimethacrylate (15%), cyclohexanol (48%), dodecanol (12%), and azo-bis-isobutyronitrile (1% with respect to monomers) at a temperature of 70oC.
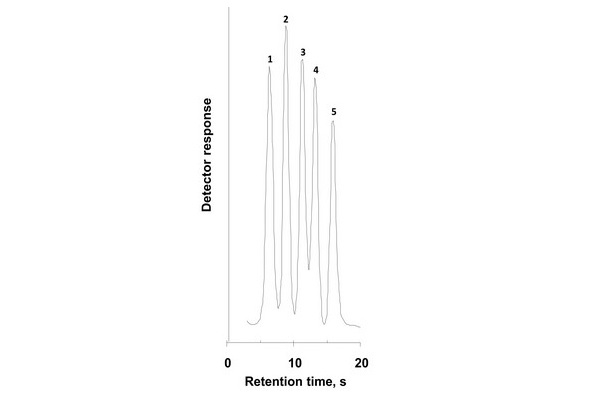
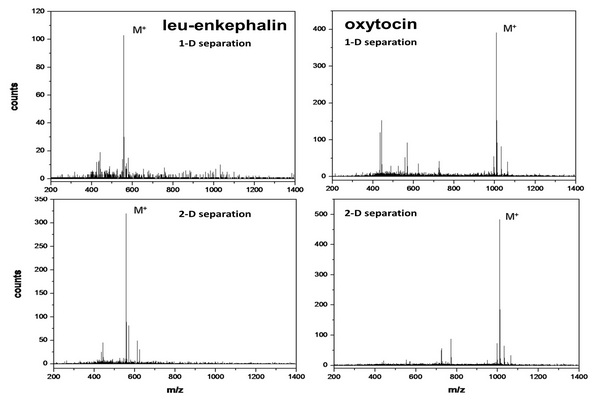
Figure 5.2
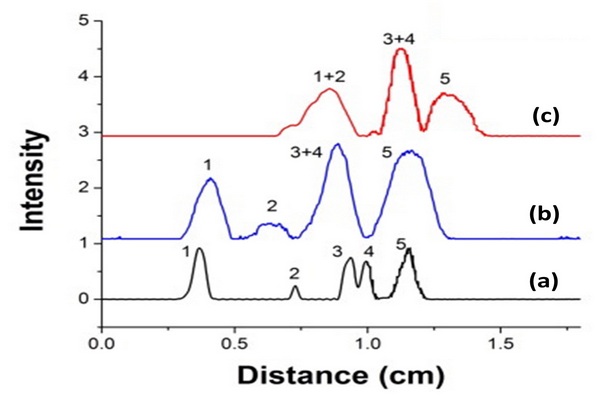
Fast reversed-phase separation of proteins. Conditions: Poly(styrene-co-divinylbenzene) monolithic column 50 x 4.6 mm I.D., mobile phase gradient 42%-90% acetonitrile in 0.15% aqueous trifluoroacetic acid in 0.35 min, flow rate 10 mL/min, UV detection at 280 nm. Peaks: ribonuclease (1), cytochrome c (2), bovine serum albumin (3), carbonic anhydrase (4), and ovalbumin (5).
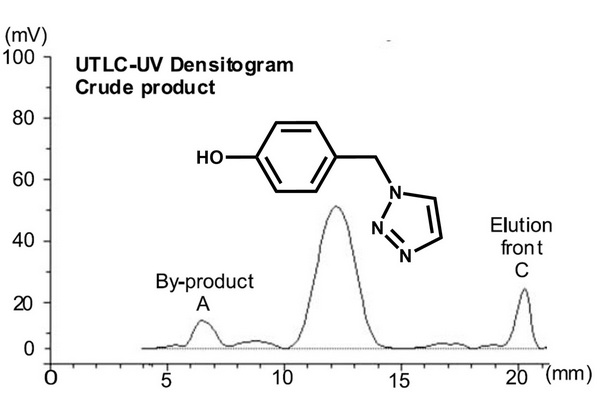
Figure 5.3
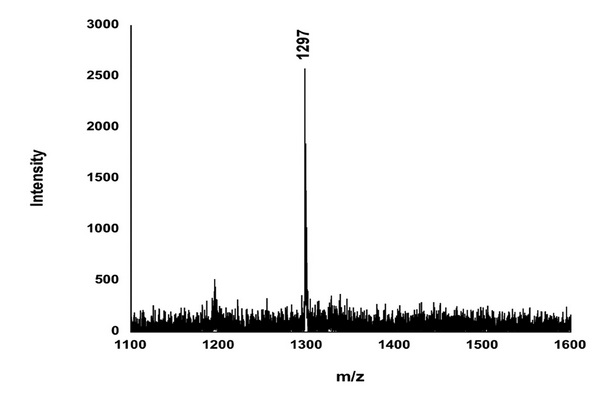
The separation of crude products from synthesis of triazole (m/z 176) using the mobile phase ethyl acetate n-hexane (1:2 v/v) with 2% acetic acid and presented as ultrathin layer chromatography (UTLC)-UV densitogram of the synthesis sample (top). Identification of peaks was achieved using atmospheric pressure matrix-assisted laser desorption/ionization (MALDI)-mass spectrometry (MS) spectra of the by-product A (m/z 369) (center) and the product (m/z 198 [M+Na]+ and m/z 107) (bottom). The main matrix ions are marked with asterisks.