Chapter 1 - Introduction - Page 1
Reversed-phase liquid chromatography (RPLC) is currently one of the most widely used separation techniques. During the last few years, some substantial improvements, such as innovative supports and instrumentation, have been brought forth to perform high-throughput analyses and highly efficient separations in RPLC (Guillarme et al., 2007b; Novakova et al., 2006). Such advances were mainly driven by the need to handle either a growing number of analyses or more complex samples.
Regarding high-throughput separations, there is a growing demand in numerous fields, including toxicology, doping, forensics, clinical chemistry, and environmental analyses, where the delivery time response must be reduced as much as possible. The pharmaceutical field, with its need for enhanced productivity and reduced costs, is certainly the main driving force for faster separations (Wren and Tchelitcheff, 2006). Because of the high number of analyses required for common pharmaceutical applications, such as purity assays, pharmacokinetic studies, and quality control, rapid analytical procedures (less than 5 min including equilibration time) are often mandatory (Al-Sayah et al., 2008).
Highly efficient separations are also necessary for many applications, including proteomics, plant extract analysis, and metabolomics, which all deal with very complex samples, such as biological samples, tryptic digests, or natural plant extracts (Grata et al., 2008; Petricoin and Liotta, 2004). With such difficult samples, conventional high-pressure liquid chromatography (HPLC) systems present some obvious limitations.
Among the different strategies used to achieve fast and high-resolution separations, ultrahigh-pressure liquid chromatography (UHPLC) has been rapidly recognized as a powerful and robust analytical tool. As shown in Fig. 1.1, the number of articles published in the fields of UHPLC and UHPLC-mass spectrometry (MS) has risen very quickly since 2004. Thus, in this chapter, the possibility to speed up and/or attain highly efficient separations will be demonstrated, using the UHPLC strategy in combination with UV as well as MS detectors. This chapter will also discuss the advantages and drawbacks of this approach, in comparison with other existing techniques.
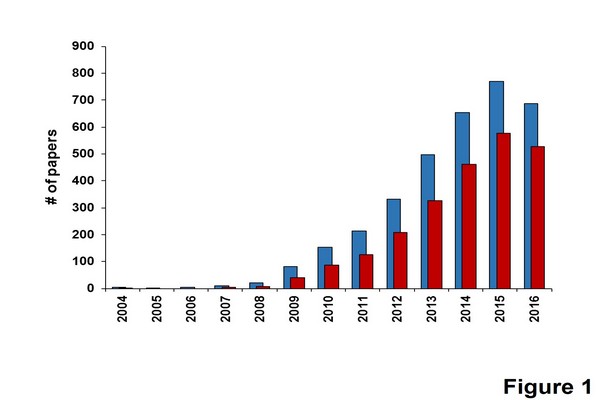
Figure 1.1
Number of papers published each year in the field of ultrahigh-pressure liquid chromatography (UHPLC) and UHPLC-mass spectrometry (MS), since 2004. Before this date, only few papers were published by Jorgenson’s and Lee’s groups. Blue bars (light gray in print versions) were obtained with keyword “UHPLC”, whereas red bars (dark gray in print versions) were obtained with an additional filter (keyword “MS”).
From Scifinder scholar 2016 search of the chemical abstracts database. Date of information gathering: August 2016.
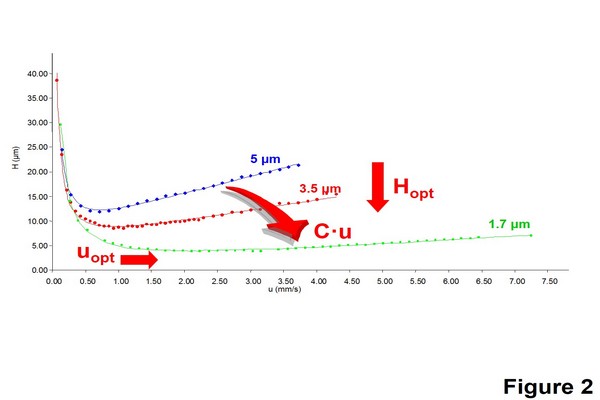
Figure 1.2
Impact of particle size reduction on the Van Deemter curves. These experimental curves were obtained with a mobile phase: H2O/MeCN (60:40 v/v), with butylparaben 25 ppm in H2O, UV detection: 254 nm, columns: XTerra RP18 4.6 mm x 150 mm, 5 um; XTerra RP 18 4.6 mm x 50 mm, 3.5 um; Acquity Shield bridged ethylsiloxane/ silica hybrid C18 2.1 mm x 50 mm, 1.7 um.
Adapted from Nguyen, D.T.T., Guillarme D., Rudaz, S., Veuthey, J.L., 2006a. Fast analysis in liquid chromatography using small particles size and ultra high pressure. J. Sep. Sci. 29, 1836-1848;
Nguyen, D.T.T., Guillarme, D., Rudaz, S., Veuthey, J.L., 2006b. Chromatographic behaviour and comparison of column packed with sub-2 um stationary phases in liquid chromatography. J. Chromatogr. A 1128, 105-113, with permission.
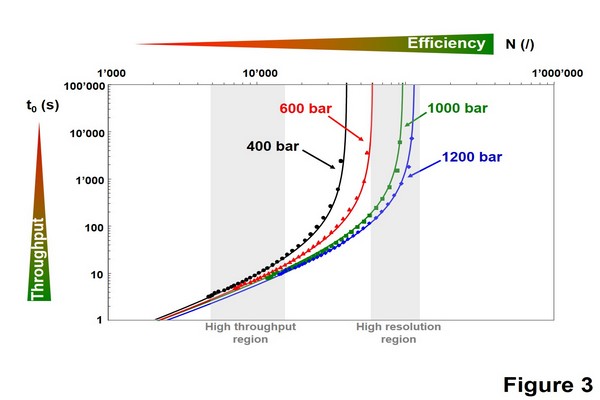
Figure 1.3
Representation illustrating the importance of increasing the maximum pressure of the system in ultrahighpressure liquid chromatography. Kinetic plots were constructed for a Waters Acquity bridged ethylsiloxane/ silica hybrid C18, 1.7 um column, considering a mobile phase viscosity of 0.85 cp (mobile phase ACN:H2O 40:60 at 30oC).
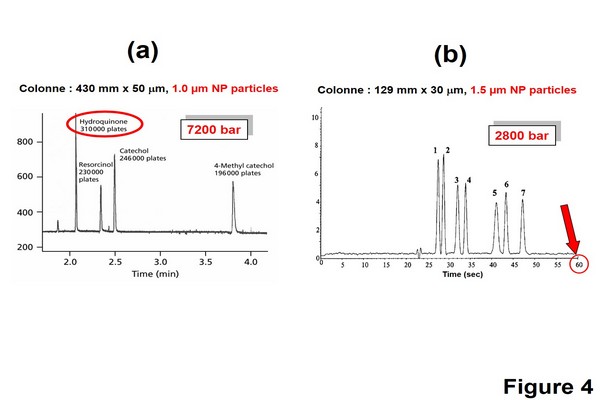
Figure 1.4
Illustration of the first ultrahigh-pressure liquid chromatography experiments made by the groups of Jorgenson et al. and Milton Lee et al. (A) Ultraefficient separation of small organic test compounds obtained; conditions: 7200 bar inlet pressure, capillary column (430 mm x 50 um I.D., packed with 1.0 um nonporous particles) (Jerkovich et al., 2003). (B) Ultrafast separation of a triazine herbicides mixture. Conditions: 2800 bar inlet pressure; capillary column (129 mm x 30 um I.D. packed with 1.5 um Kovasil mass spectrometry-H nonporous particles).
(B) Adapted from Lippert, J.A., Xin, B., Wu, N., Lee, M.L., 1999. Fast ultrahigh-pressure liquid chromatography: on-column UV and time-of-flight mass spectrometric detection. J. Microcolumn Sep. 11, 631-643, with permission.
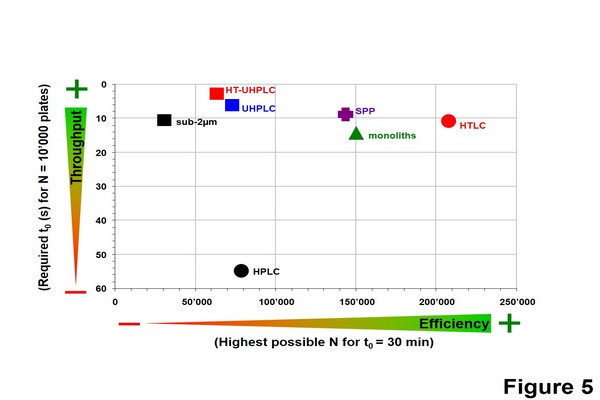
Figure 1.5
Performance comparison of liquid chromatography strategies in isocratic mode in terms of throughput (t0 for N = 10,000 plates) and maximal resolution (Nmax for t0 = 30 min) for a model compound, butylparaben with MW of 200 g/mol. The data were gathered using the kinetic plot methodology, considering a maximal pressure of 200 bar for monoliths, 400 bar for high-pressure liquid chromatography (HPLC), high-temperature liquid chromatography (HTLC) and sub-2 um, 600 bar for superficially porous particles (SPP) and 1000 bar for ultrahighpressure liquid chromatography (UHPLC) and HT-UHPLC.
Adapted from Guillarme, D., Ruta, J., Rudaz, S., Veuthey, J.-L., 2010a. New trends in fast and high resolution liquid chromatography: a critical comparison of existing approaches. Anal. Bioanal. Chem. 397, 1069-1082; Guillarme, D., Schappler, J., Rudaz, S., Veuthey, J.L., 2010b. Coupling ultrahigh pressure liquid chromatography with mass spectrometry. Trends Anal. Chem. 29, 15-27, with permission.
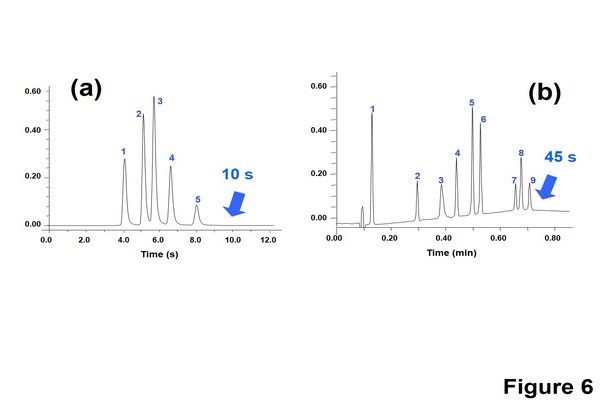
Figure 1.6
Ultrafast separations carried out in HT-ultrahigh pressure liquid chromatography. (A) Isocratic separation of various preservatives and uracil. Column: Acquity bridged ethylsiloxane/ silica hybrid (BEH) C18 (50 x 2.1 mm I.D., 1.7 um); mobile phase: water-acetonitrile (50:50%, v/v); flow rate: 1800 uL/min; temperature: 90 oC; (B) Gradient separation of several doping agents. Column: Acquity BEH Shield RP18 (50 x 2.1 mm I.D., 1.7 um); mobile phase: 0.1% formic acid in water-0.1% formic acid in acetonitrile; flow rate: 1800 uL/min; temperature: 90 oC.
Adapted from Nguyen, D.T.T., Guillarme, D., Heinisch, S., Barrioulet, M.P., Rocca, J.L., Rudaz, S., Veuthey J.L., 2007. High throughput liquid chromatography with sub-2 um particles at very high pressure and high temperature. J. Chromatogr. A 1167, 76-84, with permission.
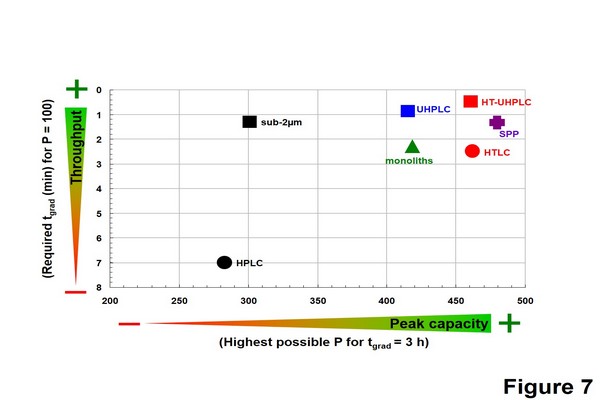
Figure 1.7
Performance comparison of liquid chromatography strategies in gradient mode in terms of throughput (tgrad for P = 100) and maximal resolution (Pmax for tgrad = 3 h) for a model compound, butylparaben with MW of 200 g/mol. The data were gathered using the kinetic plot methodology applied to gradient elution and considering the same maximal pressure as for isocratic mode. HTLC, high-temperature liquid chromatography; HT-UHPLC, hightemperature ultrahigh-pressure liquid chromatography; SPP, superficially porous particles; UHPLC, ultrahighpressure liquid chromatography.
Adapted from Guillarme, D., Ruta, J., Rudaz, S., Veuthey, J.-L., 2010a. New trends in fast and high-resolution liquid chromatography: a critical comparison of existing approaches. Anal. Bioanal. Chem. 397, 1069-1082; Guillarme, D., Schappler, J., Rudaz, S., Veuthey, J.L., 2010b. Coupling ultra-high pressure liquid chromatography with mass spectrometry. Trends Anal. Chem. 29, 15-27, with permission.
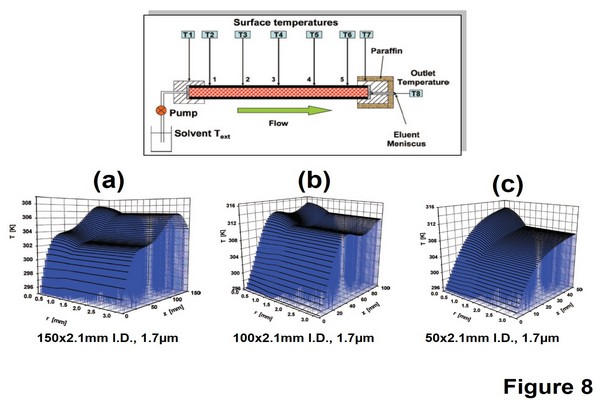
Figure 1.8
3D representation of the temperature profiles along and across various chromatographic columns (A) 150 mm x 2.1 mm (BEH) bridged ethylsiloxane/ silica hybrid-C18 column, flow rate = 0.95 mL/min, Pinlet = 979 bar. (B) 100 mm x 2.1 mm BEH-C18 column, flow rate = 1.45 mL/min, Pinlet = 1005 bar. (C) 50 mm x 2.1 mm BEH-C18 column, flow rate = 2 mL/min, Pinlet = 775 bar. The experimental setup used to measure the temperature of the surface of the column and the temperature of the liquid exiting the column outlet is also shown.
Adapted from Gritti, F., Guiochon, G., 2008. Complete temperature profiles in ultra-high-pressure liquid chromatography columns. Anal. Chem. 80, 5009 5020, with permission.
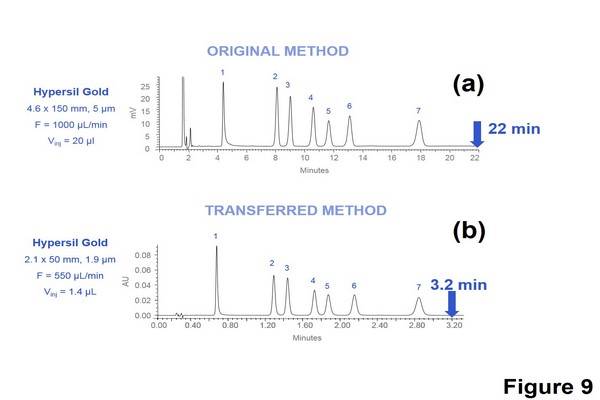
Figure 1.9
Isocratic method transfer from regular high-pressure liquid chromatography to ultrahigh-pressure liquid chromatography. Separation of a benzodiazepines mixture in isocratic mode with a mobile phase containing ACN-water (31:69, v/v) with 0.1% formic acid, T = 30oC and wavelength = 254 nm. (A) Hypersil GOLD 150 x 4.6 mm, 5 um, F = 1000 uL/min, Vinj = 20 uL. (B) Hypersil GOLD 50 x 2.1 mm, 1.9 um, F = 550 uL/min, Vinj = 1.4 uL.
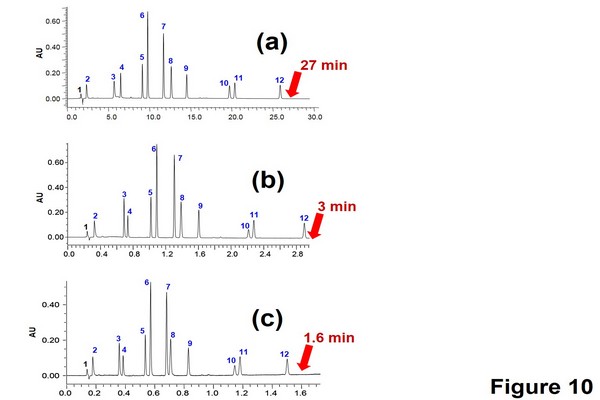
Figure 1.10
Gradient method transfer from regular high-pressure liquid chromatography (HPLC) to ultrahigh-pressure liquid chromatography (UHPLC). Separation of a pharmaceutical mixture containing the main product (6) and 11 impurities in gradient mode with HPLC and UHPLC systems: (A) original HPLC method: column: XBridge C18 150 x 4.6 mm, 5 um; flow rate: 1000 uL/min; injected volume: 20 uL; total gradient time: 45 min. (B) Transferred UHPLC method: column: Acquity bridged ethylsiloxane/ silica hybrid (BEH) C18 50 x 2.1 mm, 1.7 um; flow rate: 610 uL/min; injected volume: 1.4 uL; total gradient time: 5.1 min. (C) Transferred and optimized UHPLC method: column: Acquity BEH C18 50 x 2.1 mm, 1.7 um; flow rate: 1000 uL/min; injected volume: 1.4 uL; total gradient time: 3.1 min.
Adapted from Guillarme, D., Nguyen, D.T.T., Rudaz, S., Veuthey, J.L., 2008. Method transfer for fast liquid chromatography in pharmaceutical analysis: Application to short columns packed with small particles - Part II, Gradient separation. Eur. J. Pharm. Biopharm. 68, 430-440.
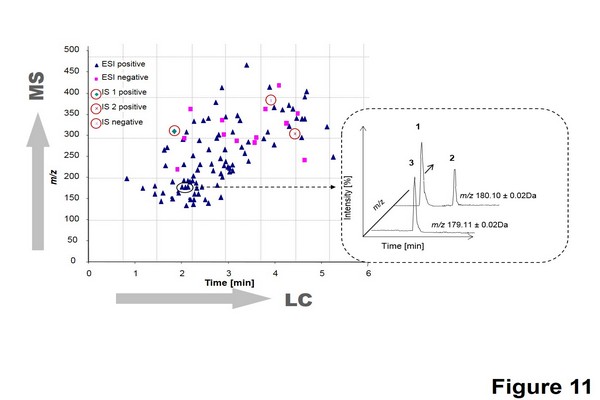
Figure 1.11
Separation of 103 doping agents in urine sample according to m/z and tR values. Data from electrospray ionization (ESI) positive and negative mode are plotted together. The three I.S are circled with a continuous line. A zone (dashed line) is magnified to show the selectivity of coupling ultrahigh-pressure liquid chromatography to the quadrupole time-of-flight mass spectrometer. In the magnified zone, the compounds (1) methylephedrine, (2) 3,4- methylenedioxyamphetamine, and (3) nikethamide are separated as a function of time, intensity, and m/z.
Adapted from Badoud, F., Grata, E., Perrenoud, L., Avois, L., Saugy, M., Rudaz, S., Veuthey, J.L., 2009. Fast analysis of doping agents in urine by ultra-high-pressure liquid chromatographyequadrupole time-of-flight mass spectrometry: I. Screening analysis. J. Chromatogr. A 1216, 4423-4433, with permission.
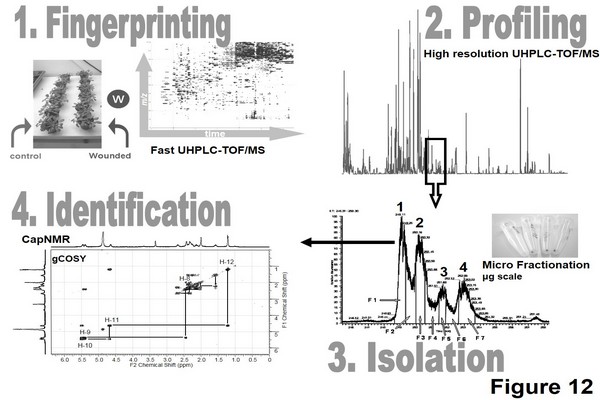
Figure 1.12
Plant metabolomics based on a four-step strategy: fingerprinting, profiling, isolation, and identification of stress biomarkers. UHPLC-TOF/MS, ultrahigh-pressure liquid chromatographyetime-of-flight mass spectrometry; capNMR, cap nuclear magnetic resonance spectroscopy.
Adapted from Guillarme, D., Ruta, J., Rudaz, S., Veuthey, J.-L., 2010a. New trends in fast and high-resolution liquid chromatography: a critical comparison of existing approaches. Anal. Bioanal. Chem. 397, 1069-1082; Guillarme, D., Schappler, J., Rudaz, S., Veuthey, J.L., 2010b. Coupling ultra-high pressure liquid chromatography with mass spectrometry. Trends Anal. Chem. 29, 15-27, with permission.












